Welcome to Texas BioGene
Texas BioGene Inc. (TBG) is a global molecular diagnostics company dedicated to the development, manufacture and marketing of nucleic acid testing kits and services. Founded in Texas, USA (1999), TBG is one of the leading providers of quality HLA Typing Kits for immune matching of bone marrow, cord blood and solid organ transplants. TBG has instituted a comprehensive quality control system and is an ISO13485 accredited manufacturer of medical devices including CE approved typing kits.
Due to it's unparalleled performance in immune matching ability, nucleic acid testing is becoming an essential tool in helping the clinician with critical transplant decisions. TBG is continually pushing to the forefront of nucleic acid testing for diagnostics. From the extraction of nucleic acids, amplification and detection of infectious diseases, genotyping and viral load testing, TBG is commited to expanding the applications of our core technology.
Quality Systems
Quality Policy
Quality is at the heart of what we do. From operations management to product design and manufacture we have instituted a comprehensive quality management system to monitor and constantly improve our products and services.
ISO 13485: A certified manufacturer of medical devices for IVD use TBG has established and manages a quality management system and maintains its effectiveness in accordance with the requirements of ISO 13485:2003, ISO 14971:2000 Standards, IVDD related standards and guidelines.
- ISO 13485 Certificate
- CE0197 Certificate- Directive for IVD manufacturers
- 510K Substantial Equivalence
- ASHI Certification for HLA Low and High Resolution Typing (NEW)
HLA Outsource Typing Service Lab
Objectives & Missions
With the goal of being one of the leaders in the industry of molecular diagnostics, we commit ourselves to innovative product development, quality-controlled production, and high-standard customer services. Through our proprietary technologies, we aim to provide diagnostic products with improved convenience, accuracy, and efficiency, which contribute significantly to disease management and human health.
Core Technology
Our expertise has been applied to the development of products for genotyping assays. The leading products include a range of HLA typing assays, featuring multiplexed design. Additionally, other genotyping and pathogen typing kits are currently in development. The platform for automated typing allows samples to be processed in a systematic manner and can be conveniently adopted by a wide range of nucleic acid testing products.
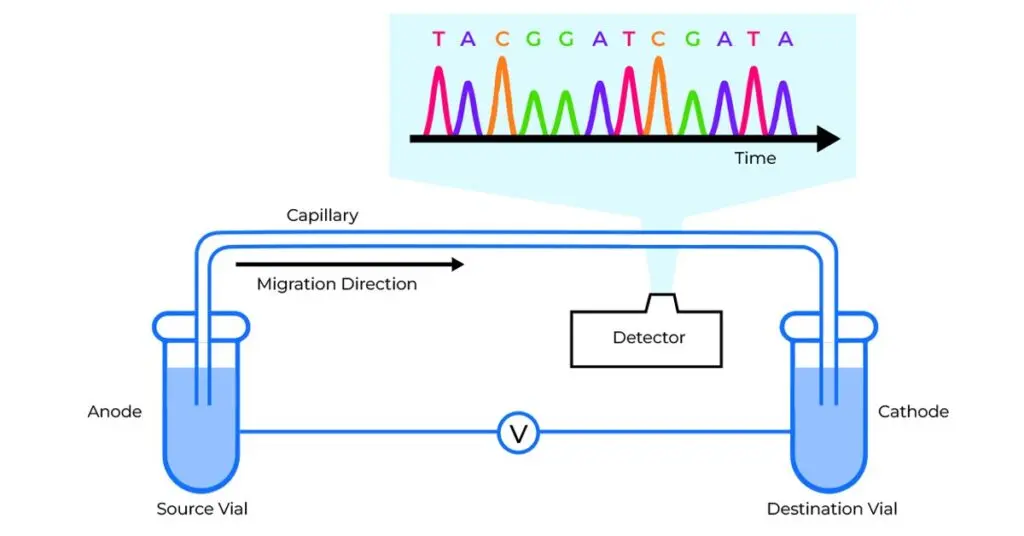
The integration of capillary electrophoresis with HLA typing is achieved through the development of a series of proprietary software for automatic sample processing and data analysis, significantly reducing the potential for human errors associated with manual handling and interpretation. State-of-the-art technologies in chemistry, molecular biology, and optoelectronics offer innovative solutions to genotyping labs, dramatically improving sensitivity, accuracy, and throughput.
Technology
Benefits of NAT Technology
Nucleic Acid Testing - Diagnostic solutions for the 21st century
Over the last decade Nucleic Acid Testing (NAT) has become the gold standard for HLA typing and infectious disease screening. NAT is particularly useful in HLA typing as it offers sequence information at the basic genetic level to the highest resolution possible therefore aiding clinicians in their determination of suitable HLA matches for transfusions and transplants. TBG utilises the SSP method whereby HLA typing is acheived through the PCR process.
With regard to infectious disease, NAT has major advantages over the sole use of serology. NAT is a more sensitive method allowing detection of lower amounts of infectious agents and therefore giving the ability to detect infections earlier than was previously possible. This ability is especially significant in blood screening. Prior to the advent of NAT being applied to blood screening, antibodies or antigens were solely used to detect contaminated blood samples. Due to the delay in production of antibodies (seroconversion) or the generation of significant amount of antigens, the window period, the time between infection and ability to detect infection, was significantly longer for serology only testing methods. With the addition of NAT methodology to traditional serology methods, the HCV window period has been cut from 66 days to 19 days. Advanced NAT methods can even detect HIV infection within 16 days of the initial infection event.

NAT is now being applied in a wider array of diagnostic tests than ever before. It is the gold standard method for detection of viral load for HIV-positive and HCV-positive patients. Viral load can be an important determinant in the treatment dosage and choice of drug therapy for patients. It may also indicate the severity of infection. Real-Time PCR provides an effective tool to establish efficacy of therapy and modify treatment when appropriate. Various PCR techniques are also used to not only identify viruses but to further identify the individual strains. This is commonly known as genotyping. This is important in the case of infection by viruses such as HPV, whereby some strains are associated with a non-malignant outcome whereas others have a high correlation with the development of cervical cancer.
At TBG, we apply PCR and Real-Time PCR techniques in our wide array of diagnostic kits and services. Nucleic acid testing is at the core of what we do as we continue to push for more accurate, more sensitive and more useful diagnostic tests for HLA typing, infectious disease detection, treatment modification and genotyping.
SSP HLA Typing
The HLA SSP Typing Kits are for determining HLA alleles using PCR techniques with sequence specific primers (SSP). Allele sequence-specific primer pairs are designed to selectively amplify target sequences which are specific to a single allele or group of alleles. This PCR-SSP method is based on the principle that only primers with completely matched sequences to the target sequences result in amplified products under controlled PCR conditions. The presence of amplified DNA fragment is a positive indication of the existence of allele specific sequence in the genomic DNA. On the other hand, mismatched primers do not generate amplicons. In addition to sequence specific primers, an internal control primer pair, which amplifies a conserved region of the house keeping gene, is included in every PCR reaction mix. The PCR product of the internal control primer pair serves as an indication of the integrity of PCR reaction.
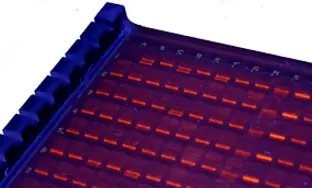
The company provides typing kits for all Class I and Class II loci and multiple combinations of HLA loci. The unique divided well design allows for more primers per PCR tray, enabling the side-by-side running of patient/donor samples on one tray. Additionally, the 96-well format typing tray can type all five loci on one tray.

HLA Typing Analysis Software
Tired of checking worksheets? Morgan™ SSPal HLA Typing Analysis Software is gel result interpretation software that has been specially designed for users of Morgan™ SSP HLA typing kits. Users just indicate the positive wells on the gel-view tick box to calculate the typing result. The software also annotates the size of specific-PCR product for double confirmation at the same time.
Users can key-in related information such as name, ID, age, ethnic and gender of the samples or patient and import raw gel pictures into the database for storage. The efficient database search function assists the user in locating their data and specific HLA types easily including parameters such as experimental conditions and gel-interpretation.
The software is designed to take into account frequent worksheet updates and users have to use the correct worksheet to interpretate the HLA typing data. Users are advised to check the worksheet SN number from the label on each kit or from the worksheet provided in each kit with the worksheet loaded in the SSPal software to see if an update has been made. Users can download updated worksheet packages from the relevant product page on the TBG website and import it into the installed SSPal software on their PC.
For example, if users want to find the latest worksheet for Morgan™ SSP HLA ABCDRDQ typing kit, they can go to HLA SSP Typing kits page then select Morgan™ SSP HLA ABCDRDQ Typing Kit to reach to Morgan™ SSP HLA ABCDRDQ Typing Kit product page. At the bottom of Morgan™ SSP HLA ABCDRDQ prdouct page users can find the latest worksheet for both manual and SSPal software interpretation.
To make a request for Morgan™ SSPal HLA Typing Analysis Software, please contact us by email or contact your local distributor.